Test for Nitrate Ions
The reactive halogen radicals were generated from nitrate photolysis in the presence of naturally abundant halide ions which facilitated the oxidation of Mn 2 aq to δ-MnO 2 nanosheets. On the amount of nitrate in the water supply and the balance of other ions in the water.

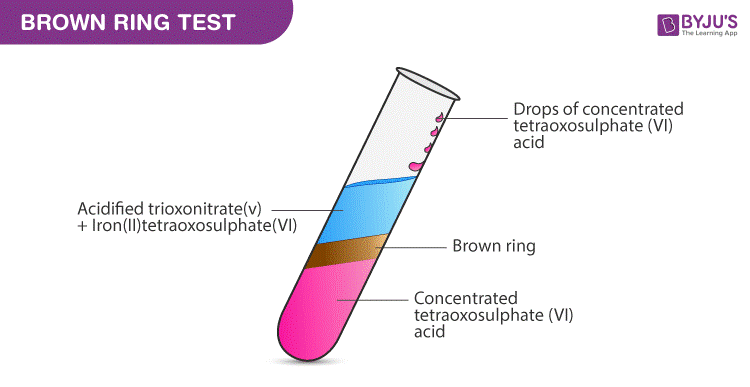
In The Ring Test Of No 3 Ion Fe 2 Ion Reduces Nitrate Ion To Nitric Oxide Which Youtube
The maximum contaminate level for Nitrate-Nitrite as N in drinking water as determined by the EPA is 10 mgL or parts per million ppm.
. However arsenate is like phosphate. The nitrate ion carries a formal charge of 1. Test for halide ions Add a few drops of dilute nitric acid then a few drops of silver nitrate solution.
Characteristics should be verified by tests conducted under established test procedures and water analysis. There are some exceptions. Silver nitrate dilute nitric acid The nitric acid reacts with and removes other ions that might also give a confusing precipitate with silver nitrate.
In this low range nitrite test nitrite ions react with sulfanilic acid to form an intermediate diazonium salt. The reduction of silver ions into metallic silver results in the formation of a silver mirror on. Observe and record the colour of any precipitate formed.
Elements in the same family make similar ions. Nitrate ions in. In new aquariums the nitrite level can gradually climb to 5 ppm or more.
Strong base neutralization reaction ii hydrogen bonding interaction between acetone and chloroform. This reacts with chronotropic acid to produce a red-orange complex directly proportional to the amount of nitrite present. Many proteins in living beings contain bound ironIII ions.
Nitrate NO 3-1 US EPA. Investigating transition metal ions. This method is used for wastewater drinking water surface water and process water.
Similarly the photolysis of DOM in the presence of halide ions can be an alternative and significant pathway to generate reactive oxygen and halogen. The accuracy of the electrode can be affected by high concentrations of chloride or bicarbonate ions in the sample water. The KH in tap water depends on the source of the water and the treatment.
Nitrate electrodes and meters are expensive compared to field kits that employ the cadmium reduction method. The toxic NH 3 is what hobbyists are concerned about but most tests give results for the total of. Enthalpy determination for i strong acid vs.
If you start from a solid it must first be dissolved in pure water. The primary issue at hand is the fact that ammonia can be present in a non-ionized form NH 3 or the ionized form NH 4 known as ammonium. This water should not be consumed until corrective action is taken.
Fluctuating pH levels can also affect the reading by the meter. So for example if you know chlorate you also know bromate and iodate too BrO3- and IO3-. Nitrite which is reduced from nitrate by gastro-intestinal microorganisms oxidizes the hemoglobins ferrous ions into ferric ions and consequently disables the oxygen transport mechanisms of the red blood cells.
However the sudden drop of Nitrate levels that follows a water change could send your fish into Osmotic Shock. The solution is acidified by adding dilute nitric acid. Electrocatalytic recycling of waste nitrate NO3 to valuable ammonia NH3 at ambient conditions is a green and appealing alternative to.
Nitrate in drinking water can lead to methemoglobinemia which is caused by nitrite in the human gastro-intestinal tract 910. Tollens Test is a chemical test used to differentiate reducing sugars from non-reducing sugars also called the silver mirror test. An ammonia test kit is one of the must-haves for every aquarium owner.
Almost all known forms of life particularly complex life require iron. Water with Nitrate-Nitrite as N less than 10 mgL is. This charge results from a combination formal charge in which each of the three oxygens carries a 2 3 charge whereas the nitrogen carries a 1 charge all these.
The body of a freshwater fish contains more ions and ionic compounds than the water. Rates of the reaction between i sodium thiosulphate and hydrochloric acid ii potassium iodate. To tell whether an unknown substance contains ironII nitrate or ironIII nitrate add a few drops of sodium hydroxide solution.
The Tollens reagent is the alkaline solution of silver nitrate AgNO 3 mixed with liquid ammonia NH 3. NITRITE NITRATE TEST What the Test Results Mean Nitrite. Nitrate is not like phosphate even though nitrogen and phosphorus are in the same group.
This test has to be done in solution. However not all ammonia tests are created equal. Those are an important subclass of the metalloproteinsExamples include oxyhemoglobin ferredoxin and the cytochromes.
Ions that end in ate have oxygen in them. The ion is the conjugate base of nitric acid consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. Nitrates are ions NO 3- and fish can gradually adapt to change in the level of ions and ionic compounds such as salts in their environment.
Equilibrium studies involving i ferric and thiocyanate ions ii CoH2O6 and chloride ions. Carbonate and Bicarbonate ions are present in municipal well and bottled spring water. The ion exchange process for example is sensitive to waters containing high TDS high sulfate and high.
Nearly all living organisms from bacteria to humans store iron as microscopic crystals 3 to 8 nm in.

Lesson Explainer Tests For Anions Nagwa

Question Video Testing For The Presence Of Nitrate Ions Using The Brown Ring Test Nagwa

No comments for "Test for Nitrate Ions"
Post a Comment